Abstract
Introduction: Novel agents are needed for multiple myeloma (MM), which remains incurable with most patients (pts) relapsing or becoming refractory to standard therapies. Talquetamab (Tal) is a bispecific antibody that binds to G protein-coupled receptor family C group 5 member D (GPRC5D), a receptor highly expressed on plasma cells with limited expression in healthy tissue, and CD3 to redirect T cells to GPRC5D-expressing MM cells. Tal monotherapy at the recommended phase 2 dose (RP2D) was well tolerated and yielded an overall response rate of 70% after 6.3 months of follow-up in pts with relapsed/refractory MM (RRMM) in the phase 1 MonumenTAL-1 study; responses were durable and continued to deepen over time. Daratumumab (Dara) is a monoclonal antibody approved for MM treatment that targets CD38 on MM cells, resulting in direct cytotoxicity of MM cells. Dara also impacts immune cell populations, ie, increasing helper and cytotoxic T cells and decreasing suppressive CD38+ immunoregulatory cells. Preclinical studies showed addition of Dara enhanced Tal-mediated lysis of MM cells, suggesting the combination may also increase clinical activity in pts with RRMM. We report initial findings for pts with RRMM who received Tal + Dara in a phase 1b multicohort study (TRIMM-2; NCT04108195).
Methods: Eligible pts (aged ≥18 years) were diagnosed with MM and had received ≥3 prior lines of therapy (including a proteasome inhibitor [PI] and immunomodulatory drug [IMiD]) or were double refractory to a PI and an IMiD. Pts were excluded if they had received an anti-CD38 therapy ≤90 days. Subcutaneous (SC) Tal and Dara were administered in 28-day cycles in different dosing cohorts (with step-up dosing for Tal). The primary objectives were to identify the RP2D of Tal in combination with Dara and to characterize the safety of Tal + Dara at the RP2D. Responses were assessed by IMWG criteria. Adverse events (AEs) were graded per CTCAE v5.0 (cytokine release syndrome [CRS] and immune effector cell-associated neurotoxicity syndrome [ICANS] graded per ASTCT guidelines).
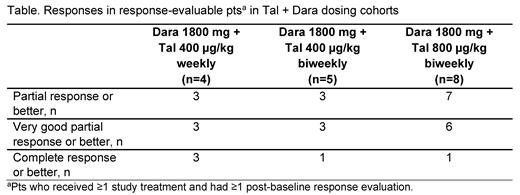
Results: As of Jul 23, 2021, 23 pts were administered SC Tal + Dara in separate cohorts: Dara 1800 mg + Tal 400 µg/kg weekly (n=8), + Tal 400 µg/kg biweekly (n=5), and + Tal 800 µg/kg biweekly (n=10). Median follow-up across the Tal + Dara cohorts was 2.9 months (range 0.3-11.2). Median age of pts treated with Tal + Dara was 68 years (range 44-81), and 52.2% were male. Pts had received a median of 6 prior lines of therapy (range 3-18); 82.6% were triple-class exposed (82.6% received prior Dara and 8.7% received prior isatuximab) and 73.9% were penta-drug exposed. Any grade AEs were reported in 95.7% of pts and grade 3/4 AEs in 78.3%. The most frequently reported AEs (≥30% across Tal + Dara cohorts) were dysgeusia (52.2%; all grade 1/2), neutropenia (39.1%; grade 3/4 30.4%), thrombocytopenia (39.1%; grade 3/4 21.7%), anemia (34.8%; grade 3/4 21.7%), CRS (34.8%; all grade 1/2), and skin exfoliation (30.4%; all grade 1/2). The median time to CRS onset was 2.5 days (range 2-4), and the median duration was 2 days (range 1-3). Infections occurred in 34.8% of pts (grade 3/4 17.4%). Skin disorders were reported in 65.2% of pts (grade 3/4 13.0%), including nail disorders in 17.4% (all grade 1/2); these events were manageable and did not lead to treatment discontinuation. Two events of ICANS were reported (a grade 1 event [concurrent with CRS] and a grade 3 event), both of which resolved and did not recur. One pt in the Dara 1800 mg + Tal 400 μg/kg biweekly cohort died due to disease progression. Responses in different dosing cohorts are shown in the Table. The median time to first response across Tal + Dara cohorts was 1.0 month (range 0.9-2.4), and median duration of response was not reached. The pharmacokinetic profile of Tal was similar to that observed in the phase 1 monotherapy study. Tal + Dara treatment resulted in proinflammatory cytokine production and T-cell activation, evidenced by interferon-γ and tumor necrosis factor-α induction and PD-1 and CD38 upregulation on peripheral T cells, respectively. Updated data with longer follow-up will be presented at the congress.
Conclusions: Tal in combination with Dara was well tolerated, with a safety profile comparable to the monotherapies, and showed promising efficacy in pts with RRMM. These findings support further clinical development of Tal + Dara combination therapy.
Chari: BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Secura Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Shattuck Labs: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Research Funding; Antengene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Millenium/Takeda: Consultancy, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi Genzyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees. Hari: Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Karyopharm: Consultancy; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Adaptive Biotech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Millenium: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene-BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau. Bahlis: Janssen: Consultancy, Honoraria; Genentech: Consultancy; BMS/Celgene: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; GlaxoSmithKline: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Mateos: Sea-Gen: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene - Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bluebird bio: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria; Oncopeptides: Honoraria. van de Donk: Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Cellectis: Research Funding. Dholaria: Janssen: Research Funding; Takeda: Research Funding; Jazz: Speakers Bureau; MEI: Research Funding; Angiocrine: Research Funding; Poseida: Research Funding; Celgene: Speakers Bureau; Pfizer: Research Funding. Garfall: Janssen: Honoraria, Research Funding; GSK: Honoraria; Novartis: Research Funding; Tmunity Therapeutics: Research Funding; Amgen: Honoraria. Goldschmidt: Novartis: Honoraria, Research Funding; Dietmar-Hopp-Foundation: Other: Grant; Sanofi: Consultancy, Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding; Takeda: Consultancy, Research Funding; MSD: Research Funding; Mundipharma: Research Funding; Molecular Partners: Research Funding; Johns Hopkins University: Other: Grant; Janssen: Consultancy, Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding; Incyte: Research Funding; GSK: Honoraria; Chugai: Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding; BMS: Consultancy, Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding; Celgene: Consultancy, Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding; Adaptive Biotechnology: Consultancy; Amgen: Consultancy, Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding. Krishnan: City of Hope Cancer Center: Current Employment; JANSSEN: Consultancy, Research Funding; BMS: Consultancy, Current equity holder in publicly-traded company, Speakers Bureau; MAGENTA: Consultancy; REGENERON: Consultancy; SANOFI: Consultancy; GSK: Consultancy; Amgen: Speakers Bureau. Martin: Janssen: Research Funding; Amgen: Research Funding; Oncopeptides: Consultancy; Sanofi: Research Funding; GlaxoSmithKline: Consultancy. Morillo Giles: Janssen: Honoraria; Takeda: Honoraria; Abbvie: Honoraria. Oriol: Oncopeptides: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Reece: BMS: Honoraria, Research Funding; GSK: Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Millennium: Research Funding; Karyopharm: Consultancy, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Sanofi: Honoraria. Rodriguez: Takeda: Consultancy, Speakers Bureau; Karyopharm: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Oncopeptides: Consultancy, Honoraria; BMS: Consultancy, Speakers Bureau. Rodriguez-Otero: Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Regeneron: Honoraria; Clínica Universidad de Navarra: Current Employment. San-Miguel: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm, Merck Sharpe & Dohme, Novartis, Regeneron, Roche, Sanofi, SecuraBio, Takeda: Consultancy, Other: Advisory board. Usmani: Array BioPharma: Consultancy, Research Funding; GSK: Consultancy, Research Funding; Celgene/BMS: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy; Amgen: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; EdoPharma: Consultancy; Merck: Consultancy, Research Funding; Sanofi: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; SkylineDX: Consultancy, Research Funding; Janssen Oncology: Consultancy, Research Funding; Takeda: Consultancy, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Research Funding. Verona: Janssen: Current Employment. Wang Lin: Janssen: Current Employment, Current holder of individual stocks in a privately-held company. Prior: Janssen: Current Employment, Current holder of individual stocks in a privately-held company. Wade: Janssen: Current Employment, Current holder of individual stocks in a privately-held company. Weiss: Janssen: Current holder of individual stocks in a privately-held company, Ended employment in the past 24 months. Goldberg: Janssen: Current Employment, Current holder of individual stocks in a privately-held company. Askari: Janssen: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal